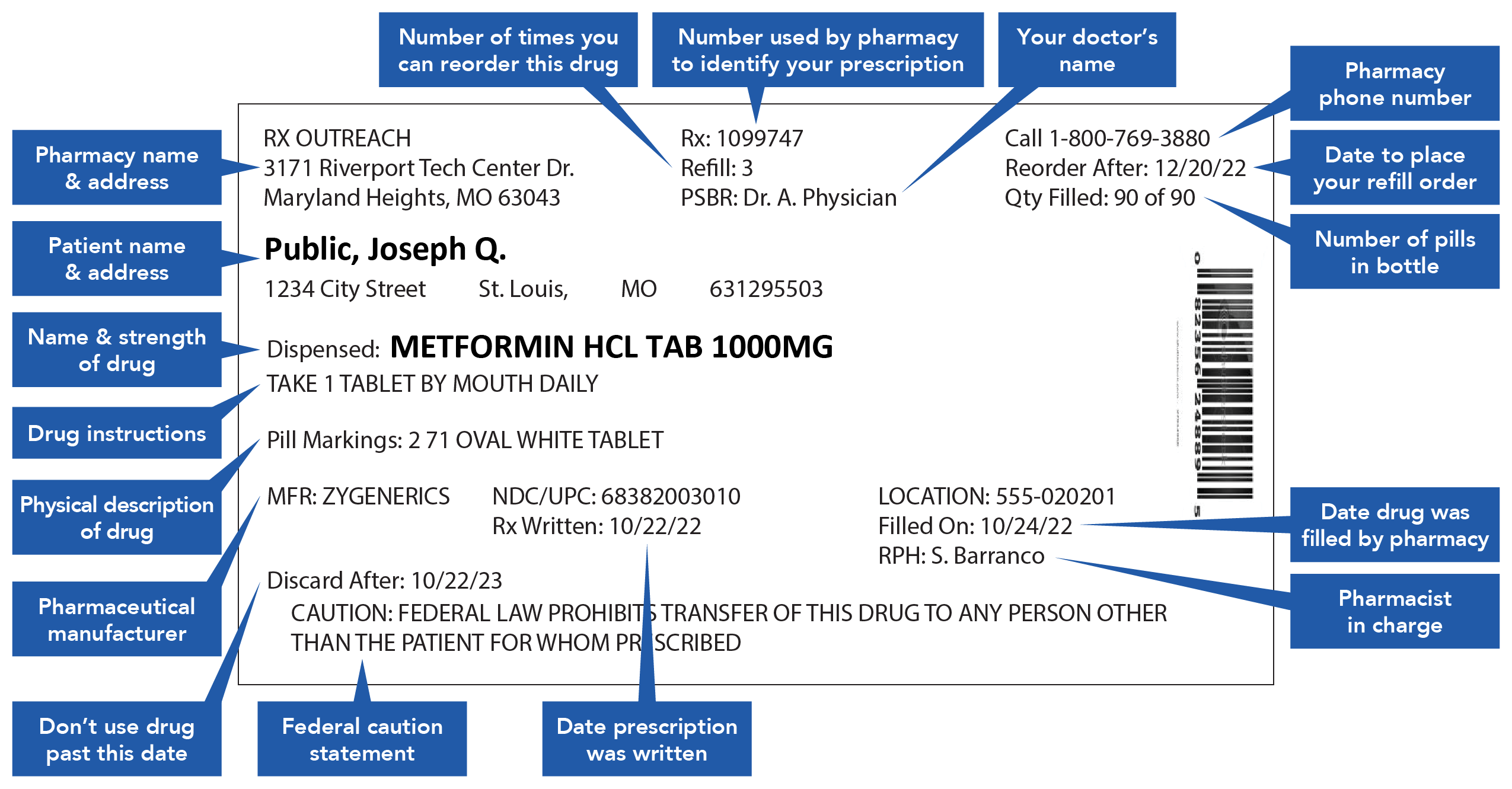
Medication Outer Label Will State . In the regulations specified in § 1.1(c) of this chapter , the term package. Discuss how the labeling for prescription medicines are. What products need pharmaceutical labeling? healthcare providers should assess medicines names, labels and packages, as well as associated devices and software,. in this guidance, the fda provides a set of principles and recommendations to drug sponsors to ensure that critical elements of a. recognize the different types of labeling for prescription medicines. According to the fda, a large section of products are. § 1.20 presence of mandatory label information.
from rxoutreach.org
What products need pharmaceutical labeling? § 1.20 presence of mandatory label information. healthcare providers should assess medicines names, labels and packages, as well as associated devices and software,. recognize the different types of labeling for prescription medicines. Discuss how the labeling for prescription medicines are. in this guidance, the fda provides a set of principles and recommendations to drug sponsors to ensure that critical elements of a. In the regulations specified in § 1.1(c) of this chapter , the term package. According to the fda, a large section of products are.
Education Understanding Prescription Medication Labels Rx Outreach
Medication Outer Label Will State In the regulations specified in § 1.1(c) of this chapter , the term package. What products need pharmaceutical labeling? § 1.20 presence of mandatory label information. in this guidance, the fda provides a set of principles and recommendations to drug sponsors to ensure that critical elements of a. recognize the different types of labeling for prescription medicines. In the regulations specified in § 1.1(c) of this chapter , the term package. According to the fda, a large section of products are. healthcare providers should assess medicines names, labels and packages, as well as associated devices and software,. Discuss how the labeling for prescription medicines are.
From drugicon.cc
Drug Labelling Designs A Comparative Study Drug Icon CC 藥物圖標 Medication Outer Label Will State in this guidance, the fda provides a set of principles and recommendations to drug sponsors to ensure that critical elements of a. According to the fda, a large section of products are. In the regulations specified in § 1.1(c) of this chapter , the term package. What products need pharmaceutical labeling? Discuss how the labeling for prescription medicines are.. Medication Outer Label Will State.
From my.clevelandclinic.org
How To Read A Prescription Label A Guide Cleveland Clinic Medication Outer Label Will State Discuss how the labeling for prescription medicines are. in this guidance, the fda provides a set of principles and recommendations to drug sponsors to ensure that critical elements of a. What products need pharmaceutical labeling? healthcare providers should assess medicines names, labels and packages, as well as associated devices and software,. According to the fda, a large section. Medication Outer Label Will State.
From www.youtube.com
How to read a medication label YouTube Medication Outer Label Will State recognize the different types of labeling for prescription medicines. What products need pharmaceutical labeling? healthcare providers should assess medicines names, labels and packages, as well as associated devices and software,. In the regulations specified in § 1.1(c) of this chapter , the term package. Discuss how the labeling for prescription medicines are. § 1.20 presence of mandatory. Medication Outer Label Will State.
From mediqueproducts.com
Medique Products The Brands That Work Medication Outer Label Will State in this guidance, the fda provides a set of principles and recommendations to drug sponsors to ensure that critical elements of a. What products need pharmaceutical labeling? In the regulations specified in § 1.1(c) of this chapter , the term package. § 1.20 presence of mandatory label information. recognize the different types of labeling for prescription medicines.. Medication Outer Label Will State.
From ardozseven.blogspot.com
30 What Information Is Required On A Medication Label Labels Database Medication Outer Label Will State According to the fda, a large section of products are. healthcare providers should assess medicines names, labels and packages, as well as associated devices and software,. recognize the different types of labeling for prescription medicines. In the regulations specified in § 1.1(c) of this chapter , the term package. in this guidance, the fda provides a set. Medication Outer Label Will State.
From www.nia.nih.gov
Taking Medicines Safely as You Age National Institute on Aging Medication Outer Label Will State In the regulations specified in § 1.1(c) of this chapter , the term package. § 1.20 presence of mandatory label information. healthcare providers should assess medicines names, labels and packages, as well as associated devices and software,. What products need pharmaceutical labeling? recognize the different types of labeling for prescription medicines. in this guidance, the fda. Medication Outer Label Will State.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Regulations Guide Artwork Flow Medication Outer Label Will State In the regulations specified in § 1.1(c) of this chapter , the term package. recognize the different types of labeling for prescription medicines. Discuss how the labeling for prescription medicines are. What products need pharmaceutical labeling? healthcare providers should assess medicines names, labels and packages, as well as associated devices and software,. in this guidance, the fda. Medication Outer Label Will State.
From healthyheels.org
Medication Label Literacy UNC Healthy Heels Medication Outer Label Will State recognize the different types of labeling for prescription medicines. § 1.20 presence of mandatory label information. healthcare providers should assess medicines names, labels and packages, as well as associated devices and software,. What products need pharmaceutical labeling? Discuss how the labeling for prescription medicines are. in this guidance, the fda provides a set of principles and. Medication Outer Label Will State.
From www.umc.edu
Medication labels University of Mississippi Medical Center Medication Outer Label Will State According to the fda, a large section of products are. Discuss how the labeling for prescription medicines are. healthcare providers should assess medicines names, labels and packages, as well as associated devices and software,. In the regulations specified in § 1.1(c) of this chapter , the term package. in this guidance, the fda provides a set of principles. Medication Outer Label Will State.
From rxoutreach.org
Education Understanding Prescription Medication Labels Rx Outreach Medication Outer Label Will State recognize the different types of labeling for prescription medicines. According to the fda, a large section of products are. healthcare providers should assess medicines names, labels and packages, as well as associated devices and software,. in this guidance, the fda provides a set of principles and recommendations to drug sponsors to ensure that critical elements of a.. Medication Outer Label Will State.
From bravenhealth.com
Learn About Your Drug Coverage Braven Health Medication Outer Label Will State recognize the different types of labeling for prescription medicines. Discuss how the labeling for prescription medicines are. What products need pharmaceutical labeling? healthcare providers should assess medicines names, labels and packages, as well as associated devices and software,. In the regulations specified in § 1.1(c) of this chapter , the term package. § 1.20 presence of mandatory. Medication Outer Label Will State.
From www.getreliefresponsibly.ca
Understanding Medicine Labels GET RELIEF RESPONSIBLY® Medication Outer Label Will State What products need pharmaceutical labeling? in this guidance, the fda provides a set of principles and recommendations to drug sponsors to ensure that critical elements of a. In the regulations specified in § 1.1(c) of this chapter , the term package. § 1.20 presence of mandatory label information. According to the fda, a large section of products are.. Medication Outer Label Will State.
From mavink.com
Sample Medication Labels Medication Outer Label Will State recognize the different types of labeling for prescription medicines. Discuss how the labeling for prescription medicines are. In the regulations specified in § 1.1(c) of this chapter , the term package. in this guidance, the fda provides a set of principles and recommendations to drug sponsors to ensure that critical elements of a. According to the fda, a. Medication Outer Label Will State.
From www.nnepc.org
How to read an overthecounter medication label Northern New England Medication Outer Label Will State What products need pharmaceutical labeling? Discuss how the labeling for prescription medicines are. In the regulations specified in § 1.1(c) of this chapter , the term package. According to the fda, a large section of products are. § 1.20 presence of mandatory label information. healthcare providers should assess medicines names, labels and packages, as well as associated devices. Medication Outer Label Will State.
From www.youtube.com
How to Read a Medication Label Nursing Skill Medication Medication Outer Label Will State According to the fda, a large section of products are. § 1.20 presence of mandatory label information. recognize the different types of labeling for prescription medicines. What products need pharmaceutical labeling? healthcare providers should assess medicines names, labels and packages, as well as associated devices and software,. In the regulations specified in § 1.1(c) of this chapter. Medication Outer Label Will State.
From animalia-life.club
Fda Drug Labeling Requirements Medication Outer Label Will State in this guidance, the fda provides a set of principles and recommendations to drug sponsors to ensure that critical elements of a. According to the fda, a large section of products are. healthcare providers should assess medicines names, labels and packages, as well as associated devices and software,. § 1.20 presence of mandatory label information. What products. Medication Outer Label Will State.
From blog.caresfield.com
Medication Labels 101 Categories, Regulations, and Best Practices Medication Outer Label Will State Discuss how the labeling for prescription medicines are. In the regulations specified in § 1.1(c) of this chapter , the term package. recognize the different types of labeling for prescription medicines. § 1.20 presence of mandatory label information. in this guidance, the fda provides a set of principles and recommendations to drug sponsors to ensure that critical. Medication Outer Label Will State.
From www.jupiterfamilypractice.com
How to Read Medication Labels for OTC Products Medication Outer Label Will State recognize the different types of labeling for prescription medicines. healthcare providers should assess medicines names, labels and packages, as well as associated devices and software,. In the regulations specified in § 1.1(c) of this chapter , the term package. Discuss how the labeling for prescription medicines are. § 1.20 presence of mandatory label information. in this. Medication Outer Label Will State.